
This is because patients with the entity of a cortisol-co-secreting type of APA (aldosterone- and cortisol-producing adenoma (A/CPA)) may present with additional clinical features, impacting on care after tumor resection and because routine diagnostic tests may become more difficult to interpret and will have to be complemented. In addition, there is one subtype of PA that is underrecognized in current reviews and guidelines and that seems worthy of more detailed discussion. However, a number of open questions remain and a controversial debate arose on the quality of diagnostic tests that we employ for our patients today ( 8, 9, 10, 11). To meet the clinical challenges adequately, a clinical practice guideline for the management of patients with suspected PA was developed recommending an algorithm for screening, confirmatory, and subtype testing (7). Later, PA was recognized to occur as a result of a heterogeneous group of disorders, including aldosterone-producing adenomas (APA), idiopathic uni- or bilateral adrenal hyperplasia, adrenocortical carcinoma (ACC), aldosterone-producing tumors of the ovary, or from inherited forms of familial hyperaldosteronism (FHA), types 1–3 ( 2, 3, 4, 5, 6).
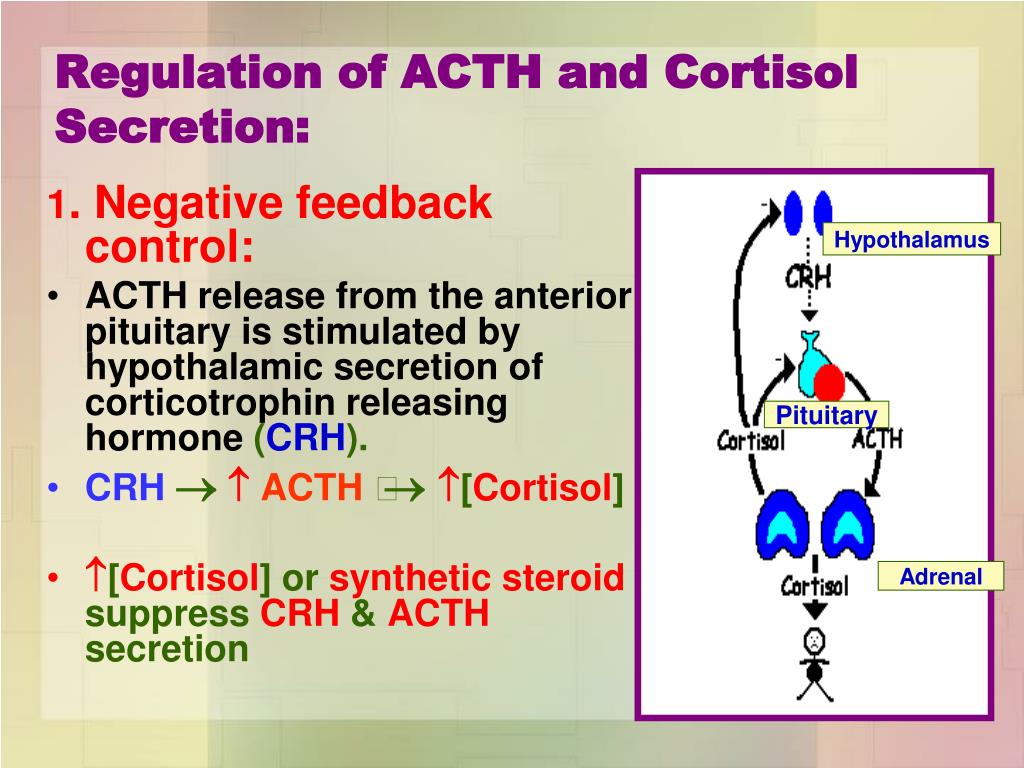
The presence of an aldosterone- and cortisol-co-secreting adrenocortical tumor should be considered if a patient has i) PA and an adenoma that is larger than 2.5 cm, ii) cortisol that is non-suppressible with overnight low-dose dexamethasone, or iii) grossly elevated serum levels of hybrid steroids, such as 18-OH-F.Ĭonn described primary aldosteronism (PA) as a syndrome of hypertension, sodium retention, and hypokalemic alkalosis that could be cured by removal of an adrenal cortical tumor and provoked by infusion of aldosterone (1). In addition, knowledge from histological and molecular studies in patients with aldosterone- and cortisol-co-secreting tumors challenges some concepts of the development of adrenal autonomy. adrenal venous sampling, screening of familial forms of PA on the basis of serum 18-hydroxy-cortisol (18-OH-F) determination, and provoke glucocorticoid deficiency after surgical removal of the tumor. Overt or subclinical glucocorticoid hypersecretion may interfere with diagnostic studies, e.g. This article reviews published data on aldosterone- and cortisol-co-secreting tumors and shows that pre-operative diagnosis of such a lesion is beneficial for patients.

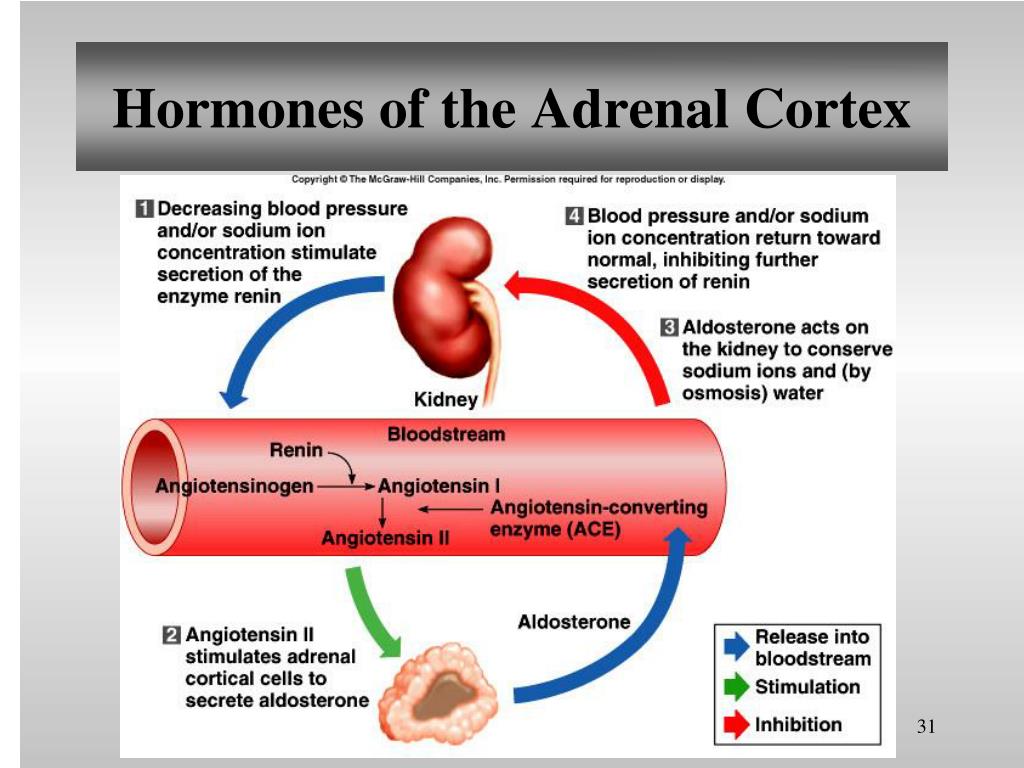
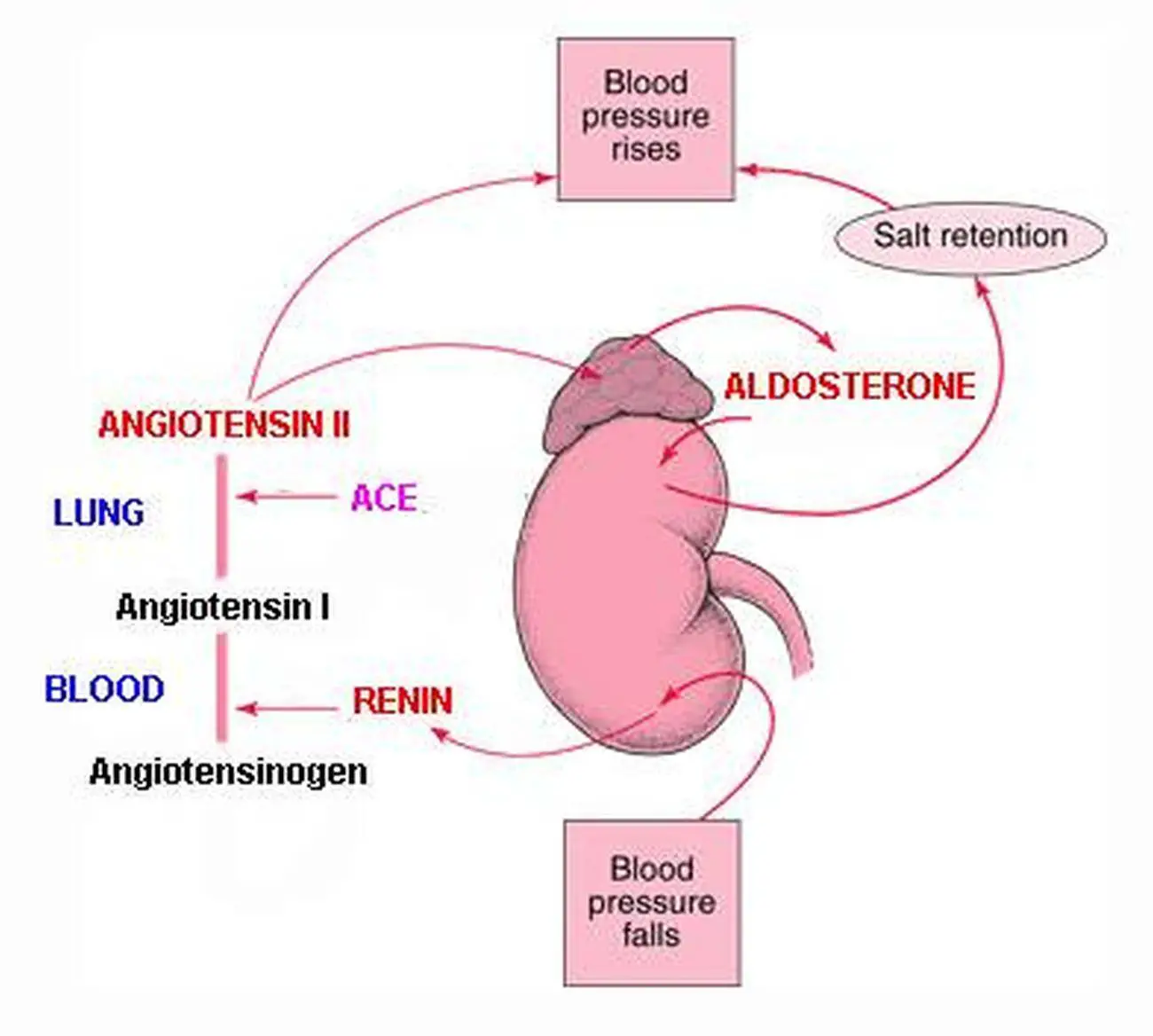
However, clinically relevant autonomous aldosterone production rarely originates in adrenal tumors, compromised of zona glomerulosa cells only. A general screening for excess secretion of other hormones is not recommended. It is further recommended to demonstrate unilateral disease because of its consequence for therapy. Current guidelines suggest proving angiotensin-independent aldosterone secretion in patients with primary aldosteronism (PA).


 0 kommentar(er)
0 kommentar(er)
